- HOME
- ABOUT US
- OUR SERVICES
- Specialized Boiler Tuning & Testing
- Pulverizer Performance & Capacity Improvements
- Pulverizer, Fan & Auxiliary Equipment Testing
- Comprehensive Boiler Inspections
- Air Emissions Testing
- Volumetric Flyash Testing
- NOx Reduction Program
- HVT Gas Sampling
- Air Flow Measurement Devices
- Comprehensive Lab & Tech Services
- Operator Training Program
- Equipment Sales
- Heat Rate Improvements
- COMMON ISSUES
- Boiler Slagging
- Pulverizer Fire or Explosions
- Pulverizer Vibrations Issues (Rumbling)
- Plugged Burner Lines
- Flame Instability
- Steam Temperature Control
- Reduced Unit Capacity
- Fireside Corrosion
- High Flyash LOI’s
- High Stack Opacity
- Raw Coal Spillage
- Pulverizer Performance and NOx Emissions
- Pulverizer Fineness
- TECHNICAL PROCEDURES
- ENVIRONMENTAL SERVICES
- CONTACT US

PULVERIZER PERFORMANCE
AND
NOX EMISSIONS
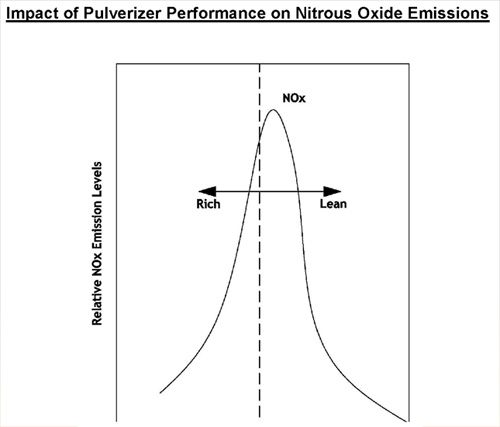
Impact of Pulverizer Performance on Nitrous Oxide Emissions
Increases in coal fineness always results in more even distribution of coal between separate burner lines. More even distribution of coal results in more precise control of burner stoichiometry that is critical to reducing NOx and achieving the best possible burner performance. Some units are more or less forgiving to fineness depending on coal quality, furnace geometry and burner configuration. In all cases, a minimum of >75% passing 200 Mesh and less than 0.5% remaining on 50 Mesh should be maintained with low NOx firing. Achieving combustion uniformity in the burner zone is one of the most critical steps to achieving target NOx emissions and optimum performance, however, it is also one of the most difficult and time consuming endeavors. Achieving combustion uniformity in the burner zone includes the balancing of fuel, primary air and secondary to each individual burner. Achieving high combustion efficiency with low NOx burners requires enhanced performance of each component in a combustion system. The combustion system includes burners, pulverizers, fans, all points of combustion air injection, boiler heating surface, unit operators, and boiler maintenance personnel.
|
 |
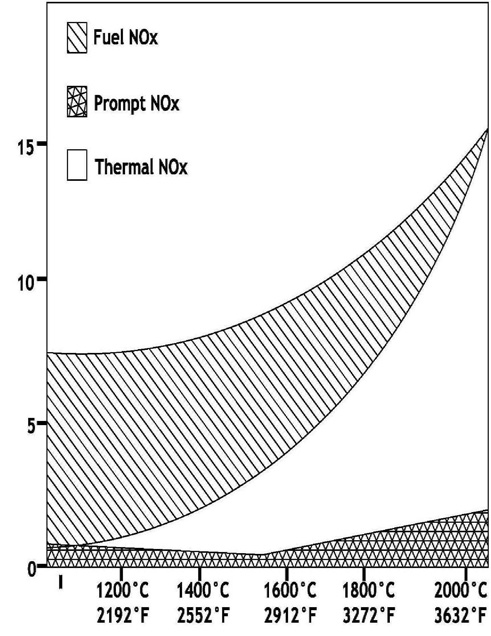
Impact of Pulverizer Performance on Nitrous Oxide Emissions
A coal fired boiler generates three types of Nitrous Oxides.
Thermal NOx. Approximately 20% of the NOx produced. It is formed from Nitrogen in combustion air. Atmospheric air utilized to provide combustion air for a coal flame is ~20.9% O2 and 78% N2. Naturally occurring Nitrogen in air is basically inert, however, temperatures above ~2800°F cause thermal dissociation of N2 and O2 allowing Nitrogen to combine with Oxygen creating primarily NO molecules. The rate of reaction that forms Thermal NOx is highly dependent upon peak flame temperature and the stoichiometric ratio in the primary combustion zone (i.e. @ burners). The maximum thermal NOx production occurs at a slightly fuel lean mixture due to the abundance of Oxygen in the hottest regions of the coal flame.
Fuel NOx. All mechanisms that form fuel NOx are not fully understood at this point in time. What is known is that Nitrogen in coal is responsible for ~80% of the total NOx formed during coal combustion. American coals are typically between ~0.25% to 1.5% nitrogen. If complete conversion of all fuel Nitrogen took place, this would result in NOx emissions of 0.4 to 2.6 Lbs./MMbtu. During the normal combustion process only
20% to 30% of the N2 content in fuel is converted to NOx. The conversion of fuel nitrogen is weakly
temperature dependent but depends strongly upon local burner stoichiometry.
Less than Optimum coal fineness, fuel balance or pulverizer airflow increases Fuel NOx
Prompt NOx. Typically insignificant; producing <5% of the total NOx formed during coal combustion. Prompt NOx is only significant in very fuel rich flames which are inherently low producers of NOx due to reduction in thermal and fuel NOx. Prompt NOx is formed in the flame zone through intermediate formation of hydrogen cyanide (HCN) followed by a subsequent oxidation of HCN to NO.
Impact of Pulverizer Performance on Nitrous Oxide Emissions
As mentioned previously, Fuel NOx is ~80% of the Nitrous Oxides formed during low NOx firing. Fuel NOx is weakly temperature dependent but strongly influenced by burner stoichiometry that is largely determined by fuel balance. The strong relationship between fuel balance and fineness, makes achieving the best possible fuel fineness and consequently, fuel balance of paramount importance.
The graph to the right illustrates the temperature dependence of the thermal, fuel and prompt NOx. Thermal NOx is easily controlled by staging air, controlling excess air and airflow management. However, fuel NOx becomes a larger percentage as thermal NOx formation is decreased.
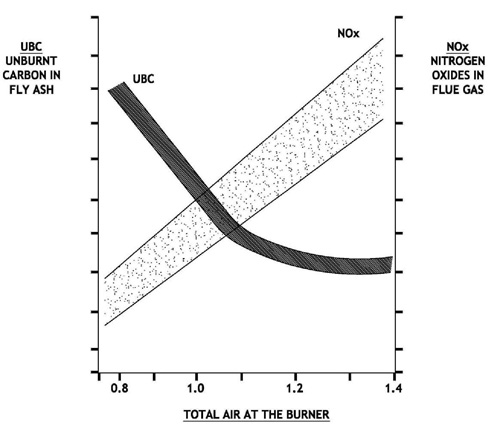
Impact of Pulverizer Performance on Nitrous Oxide Emissions
Lowering NOx by reducing free oxygen in the burner belt, or by staging combustion has been proven to reduce NOx at the expense of increased Carbon in ash and sometimes slagging tendencies or opacity challenges. Present day competitive pressures require efficient operation of large steam generators as well as environmental compliance. Minimum fly ash carbon content is achievable by implementing a comprehensive approach to combustion optimization. Actual values of carbon in ash or L.O.I. (Loss on Ignition) can vary widely, dependent on coal ash content, coal reactivity, coal volatile matter, boiler residence time and numerous boiler, as well as fuel burning equipment specifics.
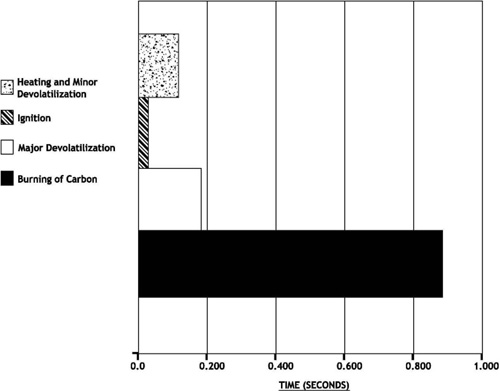
Impact of Pulverizer Performance on Nitrous Oxide Emissions
The combustion of solid carbon or “char” must be completed in a very short time. If air and carbon are not well mixed, combustion requires even more time. From the time a coal particle enters the furnace, it spends approximately 1 to 1.5 seconds above it’s ignition temperature. Most of this time is used for burning of the carbon. Coarse coal (poor fineness) results in less total surface area on the carbonaceous solids from coal. Higher fineness levels and consequently smaller particle sizing of carbonaceous solids increases the total surface area of particles to be oxidized and allows the chemical reaction of combustion to be completed more quickly. Higher fineness allows for more rapid combustion of carbon “char” in the lower furnace. This uses the available furnace retention time more effectively.
![]()
Boiler Slagging
Pulverizer Fire and Explosions
Pulverizer Vibration Issues
Reduced Unit Capacity
![]()
Click here for ICT's 5th Edition Quick Reference Guide
![]()
20 tips to help prevent slagging When firing
PRB Coal
Achieving Optimum Pulverizer Performance
ASME Coal Sampling Procedure
![]()
Envirofacts: Your One Stop Source for
Environmental Information
Mercury and Air Toxics Standards (MATS): December 21, 2011
